
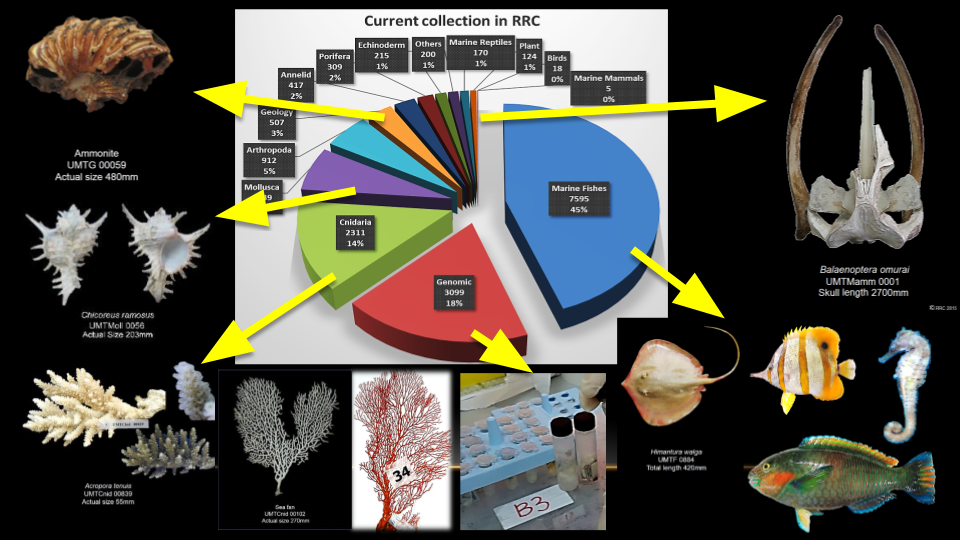
At the moment, RRC have more than 90,000 registered specimens with 90 type specimens. The collection covers all major marine phyla. In addition, there are non-marine specimens, mostly, deposited by local people who live close to RRC.
The South China Sea Repository and Reference Centre (RRC) is akin to a natural history museum, except marine and aquatic organisms are the main focus in RRC. We received specimens collected in Malaysian seas by anyone. Nonetheless, we do not merely receive all specimens.
Specimens (several individuals of the same species) that were accepted and deposited at RRC must have scientific values. For examples, new species in science or species from the area that have low information of its diversity or poisonous species like jellyfish, fire worms and crown of thorn.
After a specimen was deposited at RRC, it will be assigned a ‘Specimen Reference Number’ (ID number) and all necessary data will be recorded in the database.
All specimens then will be kept either in preservation solution (alcohol) or properly dried and then sent to one of our collection rooms. Periodically, specimens will be checked their condition to make sure they are in good condition.
Specimens in RRC represents the diversity of marine life in Malaysia, and serve as physical pieces of evidence that these organisms ever exists. These specimens can further be accessed by future researchers to check the validity of the identification and used as references to similar organisms found in other areas.
Moreover, specimens deposited can be used to determine possible alien or invasive species, used as bio-indicator or have economic potential for the nation. Some species, for example, cone shell is poisonous to human, but the venom can be used for treating pain. However, only certain cone shell has these abilities. Hence, specimens of cone shell in the repository can be used to differentiate of which cone shell is dangerous or benefited to human.
The branch of biology that deals with the study of plants, including their structure, properties, and biochemical processes

MAQIVE is under maintenance and temporarily unavailable.
Specimen loans are a privilege, and the division of specimen reserves the right to grant loan requests at its discretion. Specimens are loaned to academic or scientific institutions in the name of a recognized member of that organization.

Assoc. Prof. Dr. Izwandy Idris
INOS

Azwarina Azmi Mohd Ramasamy
INOS

Ahmad Fakhrurrazi Mokhtar
INOS

Nor Atikah Abd Manaf
INOS

Assoc. Prof. Dr. Seah Ying Giat
FPSM

Dr. Melissa Beata Martin
FSSM

Assoc. Prof. Dr. Mohd Hafiz Bokhanuddin
FSSM


Documents, posters, manuscripts, abstracts or the like, of a scientific or medical nature, which include any data, results of any clinical trial or any other information regarding or related to the Licensed Product.
South China Sea Repository & Reference Centre (RRC), Institute of Oceanography and Environment (INOS), Universiti Malaysia Terengganu (UMT), 21030 Kuala Nerus, Terengganu, Malaysia
Copyright © South China Sea Repository and Reference Centre (RRC) | Institute of Oceanography and Environment (INOS) | Universiti Malaysia Terenganu (UMT) | 2024.
All rights reserved.